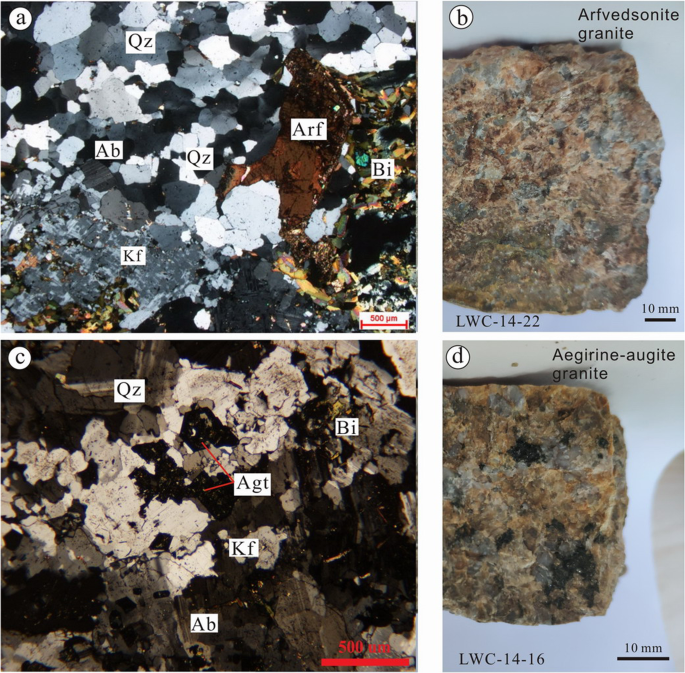
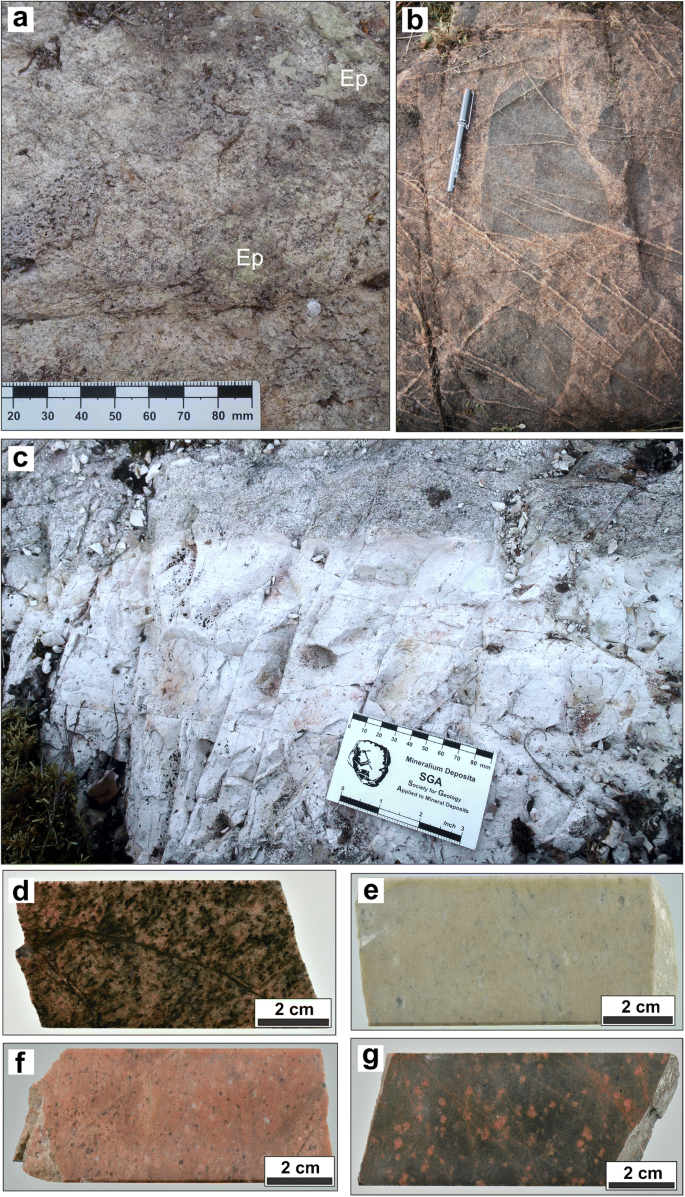
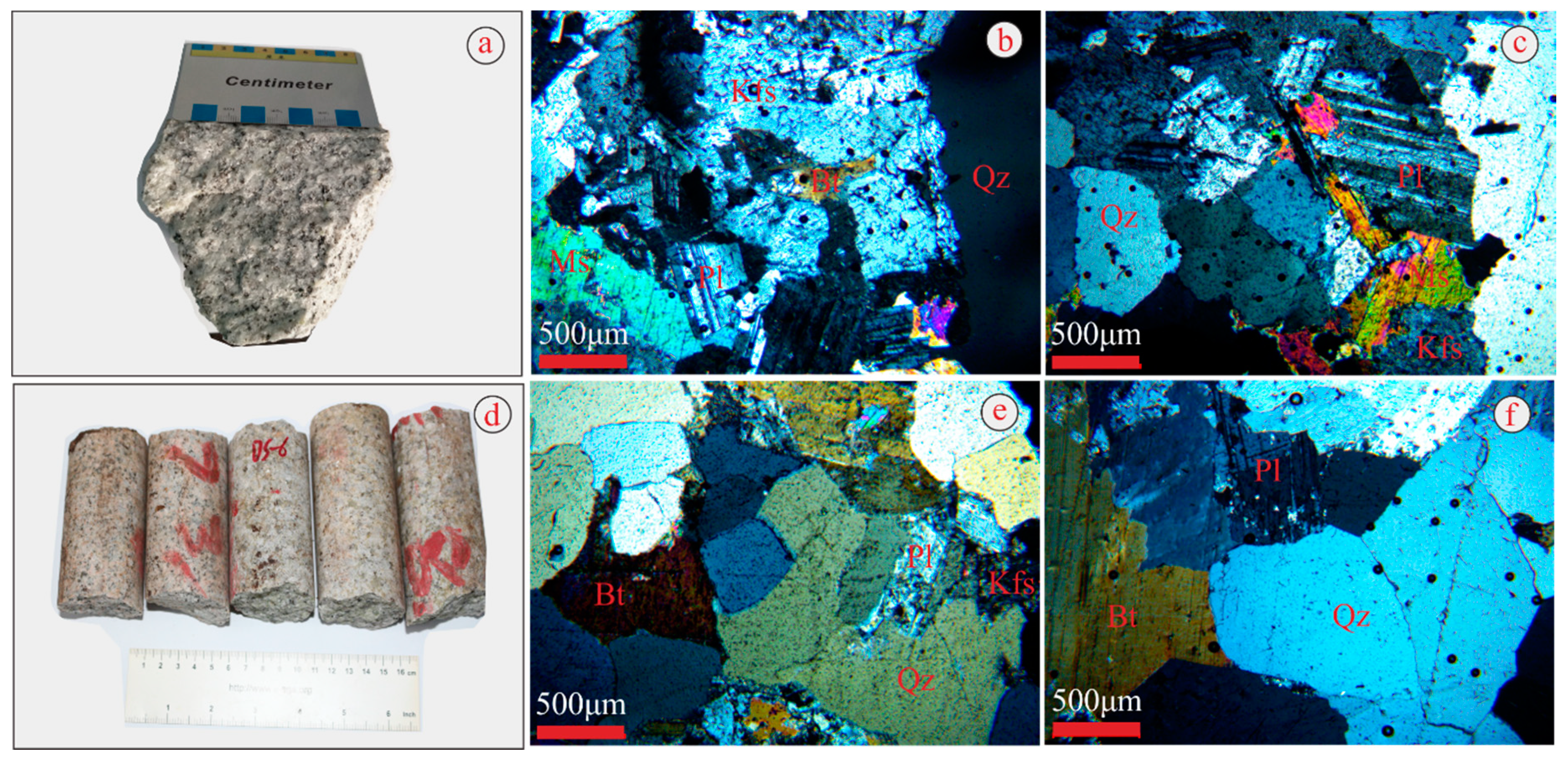
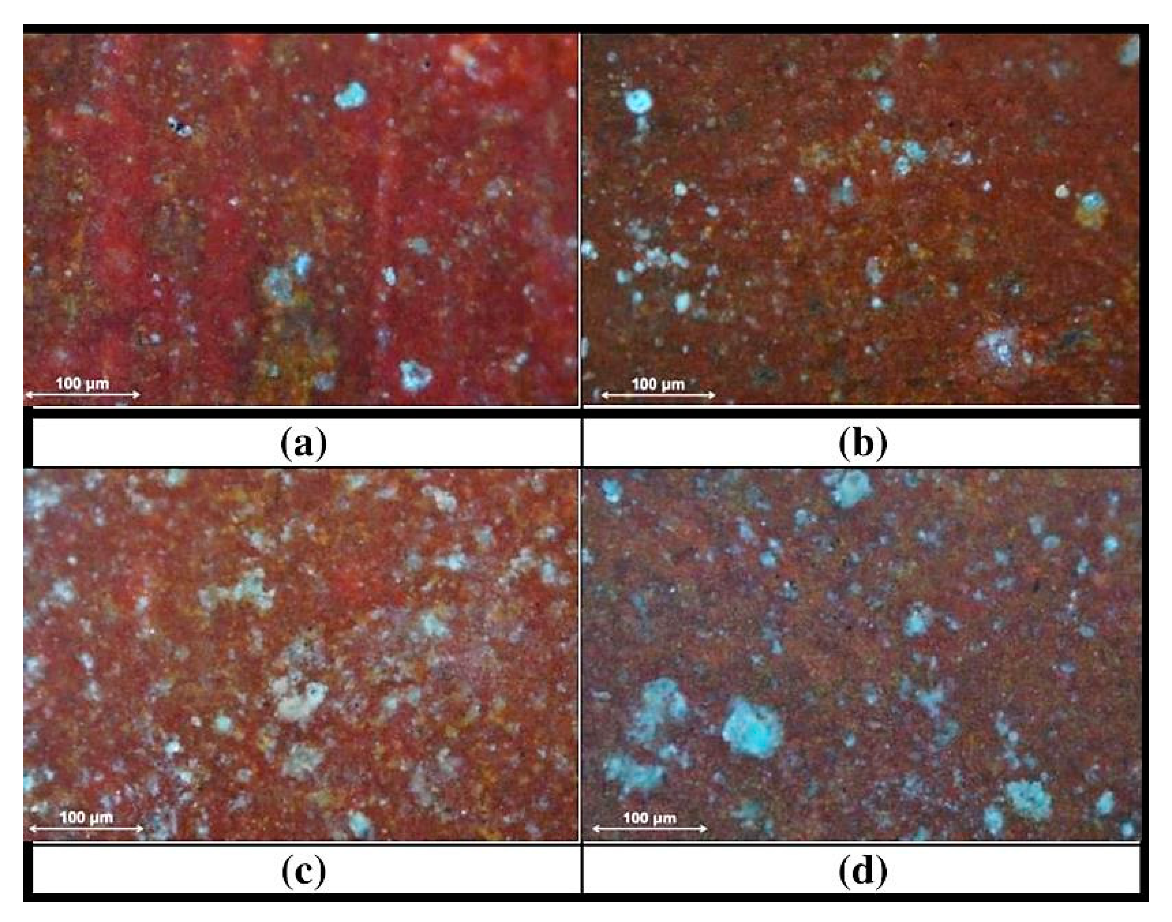
A 12 5 G Sample Of Granite Initially At 82

A 13 0 g sample of granite initially at 82 0 o c is immersed into 26 0 g of water initially at 23 0 o c.
A 12 5 g sample of granite initially at 82. Let s say we want to cool the sample down by 3 degrees. A 13 5g sample of granite initially at 80 0 c is immersed into 23 0g of water initially at 20 0 c. Determine the mass of the sample. For water cs 4 18j g c and for granite cs 0 790j g c.
For water specific heat 4 18 j g degrees celsius and for granite specific heat 0 79 j g degrees celsius. What is the final temperature of both substances when they reach thermal equilibrium. In our example it will be equal to c 63 000 j 5 kg 3 k 4 200 j. Calculate specific heat as c q mδt.
A 12 5 g sample of granite initially at 82 0 c is immersed in 25 0 g of water initially at 22 0 c. C 15 7 0 c. For water cs 4 18j g c and for granite cs 0 790j g c. What is the final temperature of both substances when they reach thermal equilibrium.
For water c s 4 18 j g c and for granite c s 0 790 j g c a. B 1 55 10 3 0 c. The answer to a 12 5 g sample of granite initially at 82 0 0c is immersed into 25 0 g of water initially at 22 0 0c. What is the final temperature of both substances when they reach thermal equilibrium.
For water cs 4 18 j g 0c and for granite cs 0 790 j g 0c. What is the final temperature of both substances when they reach thermal equilibrium. For water cs 4 18 j g oc and for granite cs 0 790 j g oc. What is the final temperature of both substances when they reach thermal equilibrium.
A 12 5 gram sample of granite initially at 82 0 oc is immersed into 25 0 grams of water initially at 22 0 oc. A 14 0g sample of granite initially at 77 0 c is immersed into 22 0g of water initially at 22 0 c. Then δt 3 k. 1 55 10 3 c c.
What is the final temperature of both substances when they reach thermal equilibrium. What is the final temperature of both substances when they reach thermal equilibrium. A 12 5 g sample of granite initially at 82 degrees celsius is immersed into 25 g of water initially at 22 degrees celsius. A 52 0 0 c.
For water cs 4 18 j oc g and for granite cs 0 790 j oc g. D 27 2 0 c. What is the final temperature of both substances when they reach thermal equilibrium. You can also go to advanced mode to type the initial and final values of temperature manually.
A 12 5 g sample of granite initially at 82 0oc is immersed into 25 0 g of water initially at 22 0oc. What is the final temperature of both substances when they reach thermal equilibrium. For water c p 4 18 j g o c and for granite c p 0 790 j g o c.